Impurities in Pharmaceuticals
Introduction:
In pharmaceuticals, impurities are unwanted substances present in a drug product other than the intended active ingredient. These substances may arise during manufacturing, processing, storage, or packaging of drugs. Impurities can originate from raw materials, chemical reactions, environmental exposure, or accidental contamination.
Organic impurities usually arise from chemical reactions, whereas inorganic substances such as heavy metals are considered impurities only when they are toxic. Depending on their origin, impurities may exist as intermediates, by-products, degradation products, or interaction products.
Sources of Impurities in Pharmacopoeial Substances:
Drug impurities may originate from raw materials, production processes, environmental exposure, packaging materials, and storage conditions. Factors such as heat, light, pH changes, humidity, reactive excipients, and prolonged storage contribute to impurity formation.
1. Raw Materials Used in Manufacturing:
Impurities may be introduced through starting materials used in drug synthesis. Poor-quality or inadequately purified raw materials may carry unwanted substances into the final product, affecting drug quality and safety.
2. Reagents Used During Production:
Reagents and solvents used during manufacturing may remain as residues if not completely removed. Residual solvents are common examples of such impurities.
3. Manufacturing Processes:
During synthesis, chemical reactions such as oxidation, reduction, hydrolysis, halogenation, and nitration may generate undesired intermediates or by-products.
- Formulation-related impurities due to interaction with excipients
- Synthetic intermediates and by-products
- Residual solvents retained after processing
- Method-related impurities caused by heat, light, pH, or reaction conditions
4. Environmental Impurities:
Industrial environments may contain gases such as sulfur dioxide, hydrogen sulfide, chemical fumes, dust, and metallic particles. These contaminants may enter drug substances during manufacturing or purification.
5. Defects in Manufacturing Process:
Incomplete reactions, improper mixing, incorrect temperature, pressure, or pH conditions may lead to formation of impurities in pharmaceutical products.
6. Manufacturing Hazards:
Contamination during processing may occur due to several hazards.
- Particulate contamination from machinery or airborne dust
- Process errors such as poor dissolution or filtration
- Cross-contamination from airborne drug particles
- Microbial contamination in liquid and topical preparations
- Packing errors including mislabeling of products
7. Storage-Related Impurities:
Improper packaging and storage conditions may lead to impurity formation due to interaction with containers, moisture, temperature, air, or light.
- Filth contamination from insects or dust
- Chemical degradation due to moisture, heat, oxidation, acids, or alkalis
- Interaction with container materials such as metals
- Physical changes like crystal growth, caking, or agglomeration
8. Accidental Substitution or Adulteration:
Accidental or deliberate substitution with substandard or toxic substances may occur. Proper labeling, segregation, and safe storage of chemicals help prevent such errors.
Effects of Impurities in Pharmacopoeial Substances:
Impurities can adversely affect drug quality, stability, safety, therapeutic efficacy, and shelf life. Toxic impurities may cause harmful effects even at low concentrations. Some impurities reduce drug potency, alter physical properties such as color, taste, and odor, or interfere with formulation compatibility.
Limit Tests:
Limit tests are analytical tests designed to detect and control small quantities of impurities present in pharmaceutical substances. These tests ensure that impurity levels remain within acceptable safety limits as defined by pharmacopoeial standards.
Limit tests are usually qualitative or semi-quantitative in nature and form an important part of pharmaceutical quality control.
Importance of Limit Tests:
- Ensure patient safety by detecting harmful impurities
- Recognize unavoidable impurities within permissible limits
- Maintain pharmacopoeial quality standards
- Prevent toxic effects caused by excessive contaminants
- Strengthen quality assurance during manufacturing
Factors Affecting Limit Tests:
- Specificity of the test for a particular impurity
- Sensitivity influenced by reagent strength, temperature, and reaction time
- Control of personal and observational errors
Common Limit Tests in Pharmaceutical Analysis
Limit Test for Chlorides:
Chloride impurity is detected by reacting chloride ions with silver nitrate in the presence of dilute nitric acid. A white turbidity of silver chloride is produced and visually compared with a standard solution.
Limit Test for Sulphates:
In acidic medium, sulphate ions react with barium chloride to form barium sulphate turbidity. Alcohol improves uniformity of turbidity, and potassium sulphate increases sensitivity.
Limit Test for Iron:
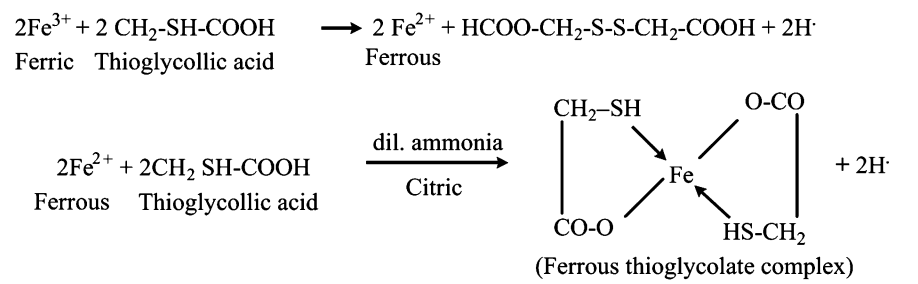
Iron reacts with thioglycolic acid in ammonium citrate buffer to produce a purple-colored ferrous complex. The color intensity is compared with a standard iron solution within a specified time.
Limit Test for Heavy Metals:
Heavy metals react with hydrogen sulphide to form colored metal sulphides. The color intensity is compared with a standard lead nitrate solution, and results are expressed in parts per million.
Limit Test for Arsenic:
In this test, arsenic is reduced to arsine gas using zinc and acid. The gas reacts with mercuric chloride paper to produce a yellow or brown stain, which is compared with a standard arsenic solution.
Summary:
Impurities are unavoidable in pharmaceutical substances but must be strictly controlled to ensure drug safety and efficacy. Understanding their sources, effects, and control through limit tests is essential for maintaining pharmacopoeial quality standards and protecting patient health.
Frequently Asked Questions (FAQs)
1. What are pharmaceutical impurities?
They are unwanted substances present in drugs other than the active ingredient.
2. Why are impurities harmful?
They may reduce drug efficacy, cause toxicity, or affect stability and safety.
3. What are limit tests?
Limit tests detect and control small quantities of impurities within acceptable limits.
4. Which impurity is tested by silver nitrate?
Chloride impurity is tested using silver nitrate.
5. Why is quality control important in pharmaceuticals?
It ensures medicines are safe, effective, stable, and compliant with pharmacopoeial standards.



